
News

Next-generation lithium-ion batteries
Lithium-ion (Li-ion) batteries are an advanced battery technology that are used in a wide range of products including personal electronics and...

EPSILON, the new generation of batteries SUPPORTED BY France 2030
As part of the France 2030 plan – Innovative solutions and technologies for batteries – SOLVIONIC’s EPSILON R&D project has just been awarded...

Applications
The product families offered by Solvionic cover a range of needs, from energy storage to surface treatments.
Our products
Innovations in both the production chain and the manufacturing process itself – Solvionic is the only company capable of producing ionic solutions ranging from liquids to salts with such a high degree of purity (>99.9%).
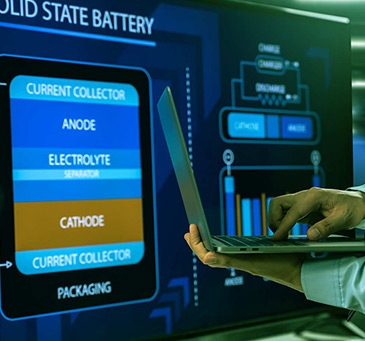
Des électrolytes ininflammables pour des batteries plus performantes et plus sûres




